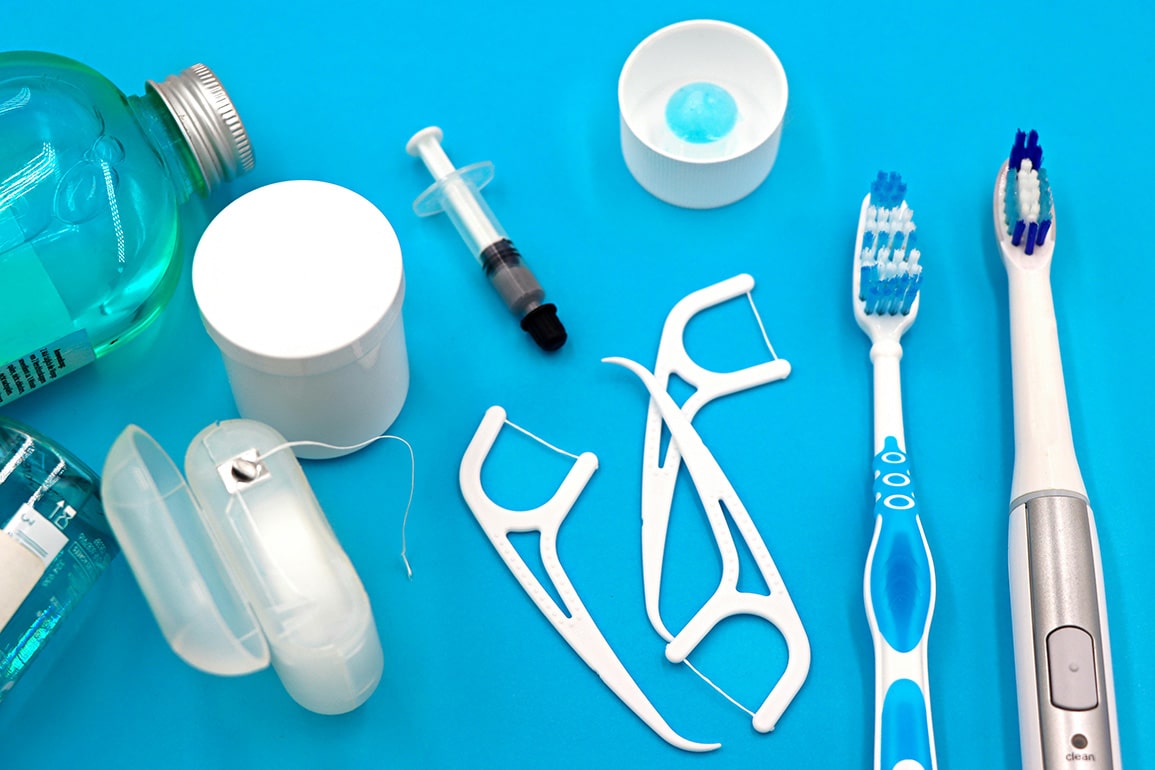
Dental care products are essential for maintaining oral hygiene and preventing dental issues. This category includes toothbrushes, toothpaste, mouthwash, dental floss, and more. Quality and user experience are crucial factors when choosing these products, as they directly impact oral health. Many dental care products are manufactured in Asian countries like China, India, and Vietnam. While these countries offer competitive pricing, there are potential quality risks associated with sourcing from Asia. Thorough Quality Control and Due Diligence are vital to ensure the products meet safety and efficacy standards. Buyers and QC professionals must prioritize these processes to guarantee trustworthy and reliable dental care products.